Nuclear medicine against cancer

The properties of lead-212
Lead-212 is an extremely rare alpha emitting radioisotope from the thorium-232 decay chain. Orano’s expertise in advanced nuclear technologies has enabled it to develop a unique process for the extraction and purification of lead-212. This rare metal, harnessed as one of the most potent therapeutic payloads against cancer cells, is being used in the development of a number of promising targeted cancer treatments, collectively known as Targeted Alpha Therapy (TAT).
Hopes for Targeted Alpha Therapy
Targeted Alpha Therapy is a technology that combines lead-212 with various biological molecules (peptides, antibodies) in order to target cancer cells. TAT can therefore selectively recognize and destroy cancer cells, while limiting the impact on surrounding healthy cells. This therapeutic approach is raising the hopes of the international medical community for progress toward less toxic and more effective treatments for patients with cancers that do not respond to existing treatments.
AlphaMedixTM, which targets neuroendocrine tumors, is Orano Med’s most advanced drug candidate
A reliable supply of lead-212
In order to manufacture and distribute its lead-212 conjugated drugs, Orano Med has invested in a unique set of facilities.
- In France, at Bessines-sur-Gartempe in the Haute-Vienne, where the Laboratoire Maurice Tubiana (LMT) produces the lead-212 precursors (radium-228 and thorium-228) from which the lead-212 doses are extracted. This facility also houses a research and development center.
- In the United States, in Plano, Texas, where the Domestic Distribution & Purification Unit (DDPU) produces lead-212 for the North American market from precursors supplied by LMT. It also produces drug substance for ongoing clinical trials to pharmaceutical industry standards.
- In Brownsburg, Indiana, the first Alpha Therapy Laboratory (ATLab) is under construction. Starting in 2024, it will be responsible for the large-scale production and distribution of lead-212 targeted therapy treatments in North America.
- In France, an ATLab is also under construction in Onnaing, near Valenciennes. This facility will also be responsible for the large-scale production of lead-212 targeted therapy treatments to meet European needs starting in 2025.
Additional ATLabs are planned to meet global needs.
ATLab Valenciennes is Europe's first industrial-scale pharmaceutical facility dedicated to the production of targeted alpha therapies with lead-212
Next steps for Orano Med
Orano Med's portfolio includes molecules developed internally or in partnership with other biotech or pharmaceutical companies in France and internationally. A dozen developments are currently underway. Among them, Orano Med is conducting two in vivo clinical trials:
- A phase 2 clinical trial on neuroendocrine tumors with AlphaMedixTM
- A phase 1 clinical trial addressing various types of solid tumors using an anti-GRPR peptide as the targeting molecule.
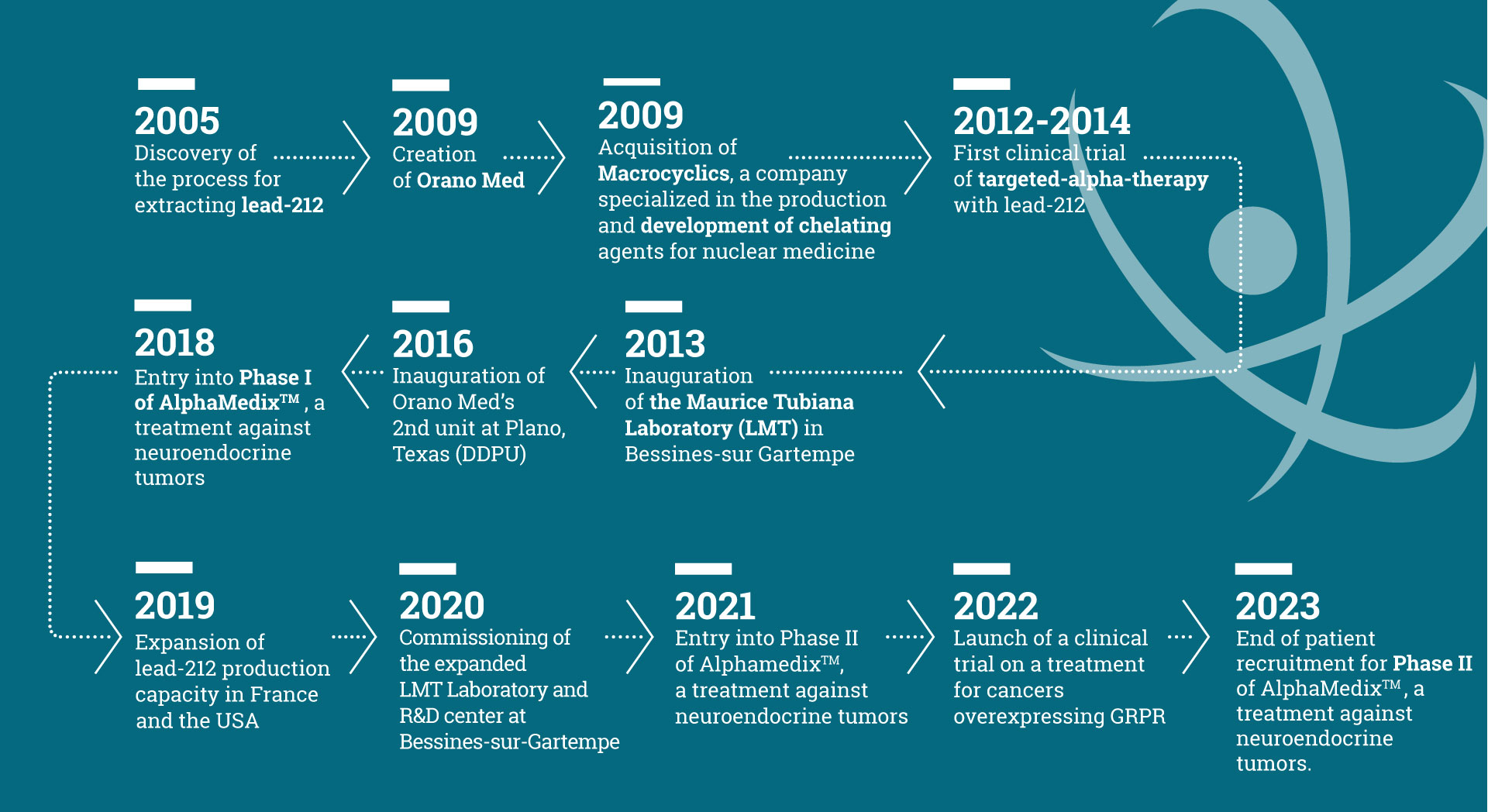
Orano Med in a few dates